A critical take on the theory after almost 30 years of its existence, featuring 6 international experts in aromatherapy and essential oil research.
In aromatherapy, when it comes to essential oil chemistry, things may look surprisingly simple. You may have heard of phrases such as:
- To prepare a wake-up blend, choose essential oils high in alcohols
- Roman chamomile essential oil prevents spasms because it is rich in esters
- You should avoid using essential oils rich in ketones because they are neurotoxic
Sounds elegant and yet sophisticated, right? These are just a few examples of claims that are based on the idea of functional groups, the only approach within aromatherapy with the status of some sort of a theory: an organised set of knowledge with predictive value.
Mastering classification of essential oil constituents into functional groups, the theory goes, we should be able to understand why certain essential oils have similar effects, and moreover to design blends precisely targeted for specific problems. Many aromatherapists have adopted such a simplified chemistry of essential oils, teaching it in courses around the world.
The problem is, the theory is wrong.
If you want a short answer, let me set the record straight right away. The functional group theory of essential oils is not an approximation of – let alone an ingenious solution to – how essential oils work. It’s simply wrong because it’s built on a false assumption. And the reason is very simple: biology cannot be reduced to chemistry.
For a long answer, keep on reading.
When I started reading books and following Facebook groups and blogs about essential oils and aromatherapy, everybody was talking about phenols, alcohols, aldehydes. It seemed to me that I was the only one who doesn’t understand what they are talking about. Which aldehyde, which phenol, ester of which alcohol and acid?
For many medicinal plants, the mechanisms of their biological effects are not yet understood. That’s why I found it extremely interesting that in aromatherapy, somehow, it is apparently possible to precisely predict the effects of every essential oil you can imagine, as long as they are analysed and the constituents classified according to their chemical structure.
Obviously, I was eager to find out more about such a fascinating subject, and moreover how to put it into practice. I soon figured that the book I would definitely get my answers from was Advanced Aromatherapy (1998) by Dr Kurt Schnaubelt. Who would have known more about the subject than a chemist with more than 30 years of experience with essential oils?

I’ll admit it: for about a month or two, I became Schnaubelt’s fan. Suddenly, everything seemed perfectly clear. You can sketch all the hefty chemistry of any essential oil into a special coordinate system and colour the functional groups represented by the predominant constituents of that oil, almost like in a colouring book. Then, according to specific needs, you combine various essential oils with mutually complementing functional groups into a synergistic personalised blend for therapeutic use. How ingeniously simple is that?
Well, the excitement was soon gone. While covering the holistic approach extensively and criticising conventional science and medicine, the book provides a very limited explanation as to why the specific groups of molecules should work in a particular way. So I started looking for the primary sources of what is today known as the functional group theory/hypothesis (Tisserand 1999). I wanted to understand where the bold assumptions came from, and after that period of excitement, I decided to get to the bottom of the matter.
WHAT EXACTLY FUNCTIONAL GROUPS ARE AND WHAT THEY HAVE TO DO WITH THE CHEMISTRY OF ESSENTIAL OILS?
In a nutshell: functional groups are small groups of atoms within molecules. They importantly contribute to their physical properties, such as solubility, melting and boiling points, as well as determine their chemical reactivity – kinds of chemical reactions they may undergo in various conditions.
Organic chemistry is essentially the chemistry of compounds that contain carbon and hydrogen atoms. Other elements bring new properties to organic compounds, oxygen being by far the most important in essential oils. Depending on how oxygen combines with carbon and hydrogen atoms, different types of molecules may form: alcohols, aldehydes, ketones, acids, esters, ethers, oxides and lactones – each characterised by the specific functional group.
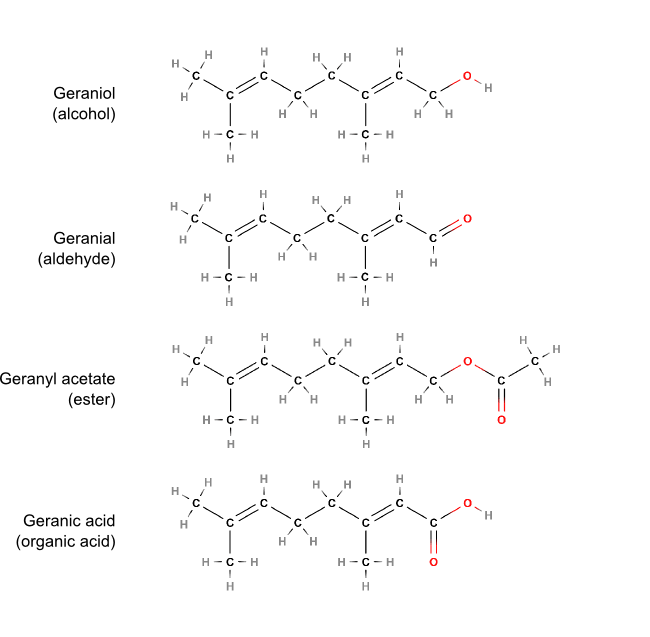 Different functional groups on the same molecular backbone (geranyl– in this case). The position and bonding of oxygen (O) to carbon (C) and hydrogen (H) gives rise to different types of molecules (note that geranic acid is not typically a constituent of essential oils, however, it is sometimes found in the hydrolats).
Different functional groups on the same molecular backbone (geranyl– in this case). The position and bonding of oxygen (O) to carbon (C) and hydrogen (H) gives rise to different types of molecules (note that geranic acid is not typically a constituent of essential oils, however, it is sometimes found in the hydrolats).
Knowing your oils’ composition according to chemical families can help you predict certain physical properties, or how long they will keep and whether you should store them in a refrigerator. To some degree, you may also infer on the distribution and metabolism of the constituents in the body.
However, with the recent essential oil chemistry hype, it may seem to a newcomer that you can’t even smell an oil without knowing how to draw a cyclic monoterpene. It’s important to understand that classification of compounds according to their chemical structure is not something new or exclusive to aromatherapy. It’s the basis for studying the biochemistry of plant volatiles: how they continuously transform from one to another in different parts of cells and plant organs, like in a giant recycling assembly system, and how various factors influence these transformations.
Chemistry also concerns changes of essential oils during and after distillation, as well as analytics. But as soon as individual constituents enter the body, we rather talk about their pharmacokinetics and pharmacodynamics.

FUNCTIONAL GROUP THEORY
Let’s take a closer look at the functional group theory of essential oils. It’s based on the assumption that compounds bearing same functional groups have similar chemical properties and will, therefore, exert similar biological effects.
Alcohols, for example, are supposed to have antimicrobial and stimulating properties, esters are anti-inflammatory, spasmolytic and soothing, while ketones have excellent mucolytic, regenerative, analgesic and antiviral properties.
The functional group theory has been used for:
- Predicting therapeutic benefits of single essential oils and blends
- Predicting potential toxicity (e.g., ketones are supposed to be neurotoxic, and thus extra caution is needed when using essential oils that contain them)
According to Daniel Pénoël MD (1999), a French medical doctor and naturopath, classification of thousands of compounds into functional groups is a vital step towards understanding therapeutic potential of essential oils, and should thus be the key part of training for their clinical application. Is that really the case?
A BRIEF HISTORY
Probably the first attempt to classify essential oils according to their chemical structure was made by Eugene Charabot and Justin Dupont in the 1920s (Gattefossé 1937/1993). Moreover, they hypothesised that biological effects – including smell perception – are determined by the presence of specific functional groups. They suggested 11 families of essential oils, based on the dominant functional groups.
René-Maurice Gattefossé, the pioneer of aromatherapy who researched clinical application of essential oils, further elaborated on this idea. He suggested classification without regard to terpenes, which would mask or denature properties of other constituents. In his book Aromatherapie (1937/1993) he recommends using deterpenised essential oils as most potent (which is against the today’s standard in aromatherapy).
The idea that structure determines function lived on and was put into clinical practice by researchers and medical practitioners such as Jean Valnet MD and Dr Paul Belaiche (known for using the aromatogram for treatment of microbial infections), who published Traité de phytothérapie et d’aromathérapie in 1979.
FRANCHOMME’S EXPERIMENT
Pierre Franchomme, a French naturopath, conducted a series of experiments in the 1980’s. In co-operation with Jean Mars, he devised a measuring system where he dispersed aerosols (tiny droplets) of isolated essential oil constituents on a charged electrode. By measuring changes in electric current, he deduced their degree of ionisation, the ability to gain or lose electric charge (Franchomme and Pénoël 1990/2001).
Depending on the direction of electric current obtained, the compounds were assigned as either positive (electron accepting) or negative (electron donating). The positioning of compounds with same functional groups to a confined area on a two-dimensional diagram, where the vertical axis represents the degree and direction of ionisation, and the horizontal axis represents the degree of polarity, was for the authors a proof that they have similar properties.

Nucleophiles, compounds that donate their electrons to form chemical bonds, are supposed to be soothing, calming, analgesic, anti-inflammatory, and generally deactivating. Electrophiles, compounds that are more likely to accept electrons, are on the other hand supposed to act in an activating and energising manner.
NOTE: Despite my efforts, I couldn’t find an explanation for how these two main types of activities derive from the measured electrical properties. Also, the terms nucleophiles and electrophiles may not be appropriate to describe these electrical properties. Phenols, for example, may also act as nucleophiles, and aldehydes may act as electrophiles – it depends on the site of chemical reaction.
Franchomme and Pénoël merged experimental findings with their experience from clinical practice and showcased them in a comprehensive book L’aromathérapy exactement, published in 1990 but never translated into English. They introduced a whole system of essential oils’ therapeutic application (referred to as the scientific aromatherapy), which is represented by the handy structure-effect diagram.
Pénoël influenced Schnaubelt, who elaborated on the idea in the United States and published Advanced Aromatherapy (mentioned earlier). In this book, he graphically represented the composition and therapeutic action of common essential oils in line with the functional group theory. The approach was further adopted by Shirley and Len Price in the United Kingdom and by many others (Peace Rhind 2012).
SHORTCOMINGS OF THE THEORY
The basic problem of the theory is that it generalises the physicochemical properties of the molecules to biological effects. Although this approach is often adopted as part of the holistic aromatherapy practice and education, it is – ironically – entirely reductionist.
Why? Because it extrapolates properties at the lower domain of organisation (physicochemical properties) to explain higher-level phenomena (biological effects). This is, by definition, reductionism in natural sciences (Felz et al. 2006, Looijen 1999).
The majority of biological effects are not determined by the molecules that trigger them directly as such but via different steps (often many) of molecular recognition and signalling. A particular active compound can cause different effects in different types of cells, depending on what is going on in a cell and which processes get activated after its binding to target proteins. In other words, biological effects are context-dependent.
Let’s take an example. Linalool, one of the most common and well-researched essential oil constituents, can reduce neural activity in the central nervous system – by binding to certain receptors that regulate the release of neurotransmitters, as well as by direct action on voltage-gated channels. In immune cells, however, it can reduce the synthesis of inflammation factors. And in liver cells, it is presumed to affect the production of triglycerides, including cholesterol. These are only three of 12 biological effects of linalool so far observed, and the proposed mechanisms are entirely different (Aprotosoaie et al. 2014).
Let’s take another example from the opposite perspective. 1,8-cineol (a monoterpene ether/oxide), linalool (a monoterpene alcohol), eugenol (a phenylpropanoid) and α-pinene (a monoterpene) are known to suppress the NF-κB transcription factor, thus exhibiting anti-inflammatory potential (Aprotosoaie et al. 2014, Greiner et al. 2013, Neves et al. 2010, Saad et al. 2013) – yet each belongs to a different chemical group. In a not so recent review, Salminen and co-workers (2008) list a total of 43 compounds of terpenic origin, hypothesised to inhibit the NF-κB signalling.
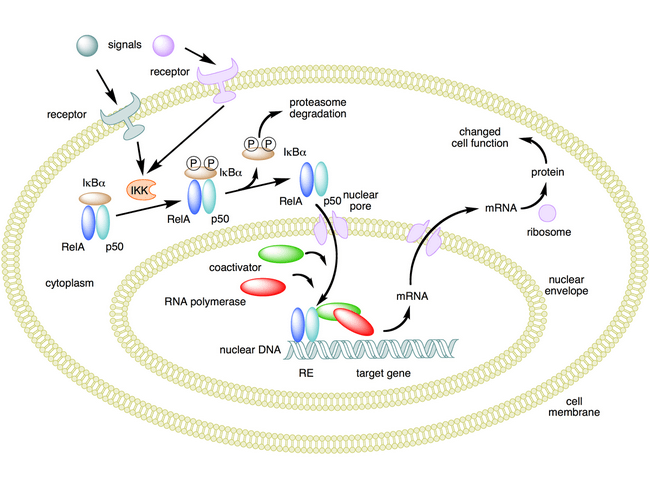
The NF-κB (nuclear factor-kappa B) pathway is a major regulatory hub in cellular processes. It controls the expression of over 500 genes involved in many physiological responses, including immunomodulation, cell adhesion and differentiation, inflammation, oxidative stress responses, carcinogenesis, and apoptosis (Gupta et al. 2010). Thus depending on the context in which a variety of potential activators and inhibitors regulate this pathway, many different biological outcomes are possible, in addition to anti-inflammatory action.
It should be evident from cases like this that chemical structure no longer has any significance for the specificity of biological effects. That’s why it is the biological – or holistic if you prefer – part of the story that the theory misses. Sure, it may appear holistic as it aims to integrate the physical and mental effects of essential oils, but the underlying assumption on which it stands is not.
Even if leaving reductionism aside, the theory is at best overgeneralising if used to predict therapeutic benefits of essential oils. Some key points:
• It is based on findings from a single set of experiments, whose results were never published in a scientific journal or repeated independently in more than 20 years.
• There is no explanation of how the two main types of biological activity – activating/energising and deactivating/suppressing – are derived from the two types of compounds, electrophiles and nucleophiles (apart from an intuitive appeal to the Traditional Chinese Medicine and the yin-yang duality).
• There are many false assumptions due to overgeneralisation concerning toxicity and therapeutic potential (not all ketones are neurotoxic, alcohols uplifting, etc.). In some cases, same types of compounds can indeed cause similar effects due to structural similarity and direct physiological mechanism. A well-known example is the antimicrobial action of phenols via cell membrane interaction and disruption of ionic balance. But there are far too many ‘exceptions’ to rely on the ‘rules’.
• The theory says nothing about the pharmacological potency of constituents since it focuses on whole groups. The predominant constituents won’t necessarily cause the strongest effects, and minor compounds can cause significant biological effects. To be familiar only with the quantity and distribution of the dominant chemical groups, without knowing the particular compounds, would be similar to picking a book by its cover.
Some of those shortcomings were already pointed out in the first critical response to the theory by Martin Watt (1995). A few years later, Tisserand (1999) challenged the authors of the theory to provide empirical evidence. Both Schnaubelt (2000) and Pénoël (1999) admitted that the theory is generalising and that there are many exceptions. But they insisted that there are still more advantages than shortcomings, emphasising the importance of having an orderly system for a better overview of therapeutic potential.
Chemistry education is undoubtedly useful, but chemistry alone cannot account for all the complexity in biological systems. This sort of approach may have had some value in the early days of essential oil research. Today, more and more research is available on single compounds as well as on complete essential oils, where interactions between constituents may further increase complexity. Although far from being understood, it is better to follow a scientifically sound route, rather than relying on a theoretically and experimentally weak aromatherapeutic ‘theory of everything’.
In spite of all this, the functional group approach and its visually appealing derivatives – from the structure-effect diagram to Rosemary Caddy’s (1997) pie charts, Philippe Mailhebiau’s (1996) aromatic triangles or Ruth von Braunschweig’s ovals (Werner and von Braunschweig 2006) – became very popular among aromatherapists. Many adopted it for its apparent elegance and simplicity and applied its tenets into curriculums and practice, sometimes with less critical distance than the creators themselves.
But I am not the only one who thinks the functional group approach is not a good solution. I invited experts with by far a broader range of experience than myself, to share their opinion on the relevancy of functional groups for biological outcomes, and on the status of the theory now, after almost 30 years since its inception. I would like to express my sincere gratitude for their thoughtful contributions. Here is what they say:
Robert Tisserand
Expert in aromatherapy and essential oil research, author and educator, Tisserand Institute
Functional group theory did not make sense to me in 1989 because even then the evidence did not support the theory – more correctly, a hypothesis. It makes even less sense in the light of current knowledge. The idea that the biological effect of an essential oil constituent is determined by its structure is quite reasonable, but a molecule’s functional group is only one of many factors that come into play. And, some biological properties are ubiquitous – it’s not helpful to state that aldehydes are anti-inflammatory for example, when this property is shared by molecules in every other functional group. Finally, the grid system with pH and electrical resistance as axes is based on an idea long-ago debunked. FGT is an appealing but nonsensical idea, and it’s shocking to me that it has been adopted by so many educators in the aromatherapy field. It’s time to move on.
Dr Jennifer Peace Rhind
Biologist, essential oil educator and author
Functional Group Theory was a hypothesis that afforded starting point for practitioners of aromatherapy. It was flawed in many ways, but for those who were attempting to relate molecular structure to function it was attractive, it certainly stimulated debate and that in itself is a very good thing. Time has passed, and in the intervening years numerous studies have investigated the actions and modes of action of hundreds of essential oil components – which can certainly inform those who look to chemistry when choosing oils and composing aromatherapeutic blends. Some of these studies have revealed that there is indeed functional group influence on specific biological actions e.g. antimicrobial, antioxidant, but in other cases it is clear that effects are related to the entire molecule – for example isomers often do not share specific properties. It is also striking how many researchers investigating complete essential oils conclude that their therapeutic effects are probably due to synergistic interactions.
Martin Watt
Medical herbalist, author and educator, Aromamedical
In the late 1980s UK aromatherapy schools started to embrace the teachings of two French therapists. Those two made fundamental errors with their theories on the chemistry of essential oils. They assumed that if a particular chemical occurred in an essential oil, that the group allocated to it by organic chemists such as alcohols, aldehydes etc., would mean any essential oil containing that chemical would have similar therapeutic activity.
There are two main reasons why that theory is wrong.
- Most essential oils contain a hundred or more natural chemicals. The fragrance and flavour trades know that some of these chemicals have powerful activity at just a few parts per billion. For example, orange oils can have 95% of a chemical called d-Iimonene. That chemical contributes little to the fragrance or flavour of orange oil. All the magic molecules are in the remaining 5% of the oil. Detailed analysis of this oil can run into several pages with numerous chemicals all acting together to give orange oils their characteristic taste and fragrance. Therefore to teach that the action of orange oil is due to it containing a single terpene is incorrect, but that is how these chemical classifications are still taught.
- How the chemistry of an essential oil can affect the body depends on how it is used. For example, if someone consumes peppermint oil it will have the well acknowledged actions of the main chemicals in the oil. However, if that same oil is used externally then the fragrance of the oil can have different actions via the olfactory system. Therefore to make sweeping generalisations of therapeutic activity based on man made classifications of chemical groups is not only wrong it is very dangerous.
Marco Valussi
Medical herbalist, author, educator and distillation expert, Gadoi
I first encountered the FGT in the mid ’90, while studying aromatherapy and herbal medicine in London. Being trained in chemistry, at the time the idea of using a chemical framework to understand the activities of the essential oils seemed a perfectly good idea, although the attempt to make parallels with TCM or other traditional medicine frameworks didn’t make much historical or scientific sense. However, the scientific weaknesses of FGT become apparent very soon, and they remain the same nowadays. FGT is at the same time too simplistic, too generalized and too rigid to be really useful.
The questions we should ask of it are: can it explain what we already know about essential oils and their components? Can it predict activities in new essential oils, or new activities in know essential oils? In both cases the answer is no, as the scientific literature clearly shows. And this should not come as a surprise: in essence FGT is just an extremely simplified and empirically weak version of the field of Structure-Activity Relationship (SAR), and anyone working in SAR knows how tentative and incomplete any conclusion we can reach with it is.
Thus FGT is not a useful research tool, but I would maintain that it is also a bad teaching instrument. It makes false generalizations that are only superficially scientific, and this gives the wrong message to students, who should instead cultivate critical thinking and learn about the scientific method; it suggests that we can use sweeping statements about entire groups of essential oils instead of concentrating on the difficult job of getting to know our oils well, of never stopping revising what we know given new data. This is a wrong message for students, who should from the very beginning come to realize that research is dynamic and we should always use our critical spirit. Students should instead be introduced to literature research and specifically to SAR tools, to gauge what we know and what we do not know about essential oils.
Prof Dr Ana Cristina Figueiredo
Professor and essential oil researcher at the Faculty of Sciences of the University of Lisbon
I think that the main problem is that we are dealing with biological systems. Indeed, compounds with some functional groups may have a tendency for certain biological properties, but the mixture in which they stand may have an either positive, neutral or negative influence on the final outcome. That is, we may expect that a certain mixture will have a certain property, and in the end what is measured may be different from what was expected, due to the other components of the mixture. On the other hand, the way the receiving cell or organism will metabolize the mixture is also very specific to the individual. That is what happens with anticancer drugs as well. The correct dose for one individual may be too high or too low for another one, due to metabolic specificity of the individual. That is, we try to generalize to simplify, but as with any generalization, there will always be an exception to the rule.
Dr Iris Stappen
Pharmacist, essential oil researcher at the Faculty of Life Sciences of the University of Vienna, author and educator
Biological activity of a compound cannot be predicted by its functional group. That is the problem with odors: if you synthesize a new compound – as we did for a long time several years ago – you never know what it will smell like. There are no major general rules so far (except for see below). In our opinion that is the same with the compounds’ bioactivity.
For esters you might assume that they smell sweet whereas compounds containing a nitrogen-atom might smell fishy, but these are the only rules in terms of an odor. So if you assume that a fishy odor is rather experienced as unpleasant, you might also assume that these compounds might have an activating effect, due to the fact, that unpleasant odors are mainly activating. The sweet esters therefore might be either relaxing or activating or even neutral (pleasant odor). That is dependent on the odors’ semantic value on a person and the context the odor is smelled in.
If applied on the skin you might assume that the resorption of an ester, due to its higher lipophilic property compared to an alcohol, is higher and therefore its blood-level would probably be higher. The overall pharmacological effect therefore might be more distinctive although it cannot be said what kind of effect that would be (activating? relaxing?). What I want to show is that the functional group can very well affect the resorption but not the activity per se – as far as we know.
CONCLUSION
There’s not much more to add. Indeed, the functional group approach initiated much debate over the last three decades and likely helped popularise aromatherapy. But if one is serious about the pharmacology of essential oils or other preparations, it’s time to move beyond simplistic generalisations, towards individual constituents and their interactions. This is not a step away from holism, quite the contrary.
REFERENCES
- Aprotosoaie, A.C., Hăncianu, M., Costache, I-I., Miron, A. 2014. Linalool: a review on a key odorant molecule with valuable biological properties. Review. Flavour and Fragrance Journal 29(4): 193–219.
- Feltz, B., Crommelinck, M. & Goujon, P. (Eds.). Self-organization and emergence in life sciences. Springer, 2006.
- Franchomme, P. and Pénoël, D. 1990/2001. L’aromatherapie Exactement. Limoges: Jallois.
- Gattefosse, R.M., 1937/1993. Gattefossé’s Aromatherapy, R. Tisserand, ed. Saffron Walden: CW Daniel Co.
- Greiner, J. F. W., Müller, J., Zeuner, M. T., Hauser, S., Seidel, T., Klenke, C., … & Kaltschmidt, B. 2013. 1, 8-Cineol inhibits nuclear translocation of NF-κB p65 and NF-κB-dependent transcriptional activity. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research, 1833(12), 2866-2878.
- Gupta S.C., Sundaram C., Reuter S., Aggarwal B.B. 2010. Inhibiting NF-κB activation by small molecules as a therapeutic strategy. Biochimica et Biophysica Acta 1799(10-12): 775–787
- Looijen, R. C. Holism and Reductionism in Biology and Ecology. Kluwer, 1999.
- Mailhebiau, P. 1994.La nouvelle aromathérapie: biochimie aromatique et influence psychosensorielle des odeurs. Lausanne,
- Neves, Â., Rosa, S., Gonçalves, J., Rufino, A., Judas, F., Salgueiro, L., … & Mendes, A. F. 2010. Screening of five essential oils for identification of potential inhibitors of IL-1-induced NF-κB activation and NO production in human chondrocytes: characterization of the inhibitory activity of α-pinene. Planta medica, 76(03), 303-308.
- Peace Rhind, J. 2012. Essential Oils: A Handbook for Aromatherapy Practice. Second Edition. Singing Dragon
- Pénoël, D. 1999. Medical Aromatherapy. International Journal of Aromatherapy 9(4): 162-165
- Saad, N. Y., Muller, C. D., & Lobstein, A. 2013. Major bioactivities and mechanism of action of essential oils and their components. Flavour and Fragrance Journal, 28(5), 269-279.
- Salminen A., Lehtonen M., Suuronen T., Kaarniranta K, Huuskonen J. 2008. Terpenoids: natural inhibitors of NF-κB signaling with anti-inflammatory and anticancer potential. Cellular and Molecular Life Sciences 65(19): 2979–2999.
- Schnaubelt, K. 1998. Advanced Aromatherapy. Rochester, VT: Healing Arts Press.
- Schnaubelt, K. 2000. Functional group therapy. International Journal of Aromatherapy 10(1/2): 62-63
- Tisserand, R. 1999. Editorial comment. International Journal of Aromatherapy 9(2)
- Watt, M. 1995. Where aromatherapy training is going wrong. The Aromatic Thymes 3(1): 7-8
- Werner, M., Braunschweig, R. 2006.Praxis Aromatherapie. Grundlagen – Steckbriefe – Indikationen. Karl F. Haug Verlag
Other sources:
Burfield T. 2004. http://www.users.globalnet.co.uk/~nodice/new/bookreviews/book31/book31.htm
Lis-Balchin, M. 2006. Aromatherapy science: a guide for healthcare professionals. London. Pharmaceutical press.
Marković, S. 2005. Fitoaromaterapija. Zagreb. Centar Cedrus.
Schnaubelt, K. 1999. Medical Aromatherapy: Healing with essential oils. Frog Books.
Watt M. aromamedical.org
Williams D.G. 2008. The Chemistry of Essential Oils: An Introduction for Aromatherapists, Beauticians, Retailers and Students (Second Edition). Micelle Press, Dorset.




Thank you Petra – a lovely clear article on this much-debated subject! Where to from here?
Thank you, Joy!
More in vivo studies, more clinical studies, more studies on complete oils, and more funding for those studies 🙂
And as Dr Karlsen said at the ISEO, we need to focus on the synthesis of all the research we already have.
Great article! Glad we are moving to a more ‘holistic’ understanding about how essential oils work synergistically. Results when using essential oils have always been individual, no matter the chemical properties and can vary for that same individual overtime. The same is true for pharmaceutical drugs. What works for one doesn’t work for another and results can vary overtime. Chemistry is a starting point, but is definitely reductionist. My question is, Isn’t trying to find out the synergistic effect of essential oils like trying to figure out how nature works? Can scientific research ever really figure out the mystery of nature and how it works?
Thank you for an interesting question!
I would say that studying synergistic interactions is definitely a huge step closer to understanding how things work in Nature. People all over the world have been using herbal preparations with synergistic effects, some of which were reverse-engineered (i.e. elucidated how they work) only recently, and many may follow in the future.
The concept of pharmacological synergy is relatively new, only a few decades old. But it is indeed gaining the momentum, not only in aromatherapy, as researchers are recognising the advantages of multi-compound drugs over single highly specific compounds. However, discovering synergistic and other interactions is a HUGE undertaking: there are literally thousands of constituents and unimaginable numbers of possible ways, at least theoretically, for them to interact.
I wouldn’t dare to predict too much how things will develop in the future, but in my opinion, we will never understand all the synergies there are and all the complexity of Nature.
I am so happy to have come across your website. I really appreciate the work you put into it.
Thanks, Becca, I’m glad you like it! Hope you come back for more.
Thank you for an insightful article. I am glad to know what didn’t make sense to me is because it doesn’t make sense.
Well said, Rebecca 🙂
Wow, finally some clear rational thinking about essential oils. I mean what are they but distilled plant matter. Of course it’s impossible to rapidly classify the effects of hundreds of various molecules in the body, without even starting to understand the basics of organic chemistry (stereochemistry, chirality, etc), let alone how they interact with the various receptors and proteins in the body. The science for this is just beginning in the deep life sciences let alone way at the other end of the spectrum near aromatherapy.
As a chemical engineer by training and experience, but with a deep interest and respect of the chemical capabilities of plants and herbal medicines, I just wish essential oil people would do as you have done, is to just be honest about what we know and don’t know about these complicated molecules.
If anyone has seen videos or Ted Talks on protein folding you’ll see how incredibly complicated the machinery of living organisms are, and how perhaps they may actually be driven by electrical forces and electromagnetism at the most fundamental level, not just bulk knowledge of the chemical structure of the molecules themselves.
Cheers!
Thank you for sharing your thoughts, Anil. I can only agree with what you say about the complexity of molecular interactions. And just to add, emergent properties of molecular networks make biological effects even more unpredictable.
Thank you for writing this! You have a beautiful and clear writing style. I am so glad I found your blog, explaining aspects of essential oils I was very curious about. I will keep on reading your blog.
Thank you for stopping by Rose, and welcome to the blog! 🙂 I’m very glad you like my style of writing – I’m doing my best to be clear and informative.
First, thank your paradigm.
Second, utopia moves, like the horizon. When you go sailing.
Third, I invite you to meet the aromatic-medicinal South West of Colombia (essential oils, Colombians). In other words, come to Colombia, visit us, for two weeks. And I show you the unknown universe of the Pacific-Andino-Amazonian aromas.
Just in case, I leave the contact information:
Dr. José-Vitelio Pineda Monge
Consulting
Secretario Técnico Cadena Productiva PAMCYA NARIÑO, COLOMBIA.
Whatsapp.: +57 317 489 6637
Cel.: +57 304 588 2686
http://ethnobotanyprc.blogspot.com.co/
https://plus.google.com/u/0/+PINEDACENTER/about
Facebook: Pineda Monge
Facebook: Pineda Research Center
When I first started school and prior, I could not grasp the functional group theory. It made no sense. I was very happy to read your article. So well written and explained. Thank you
Thank you Rehne. You sum up the sentiment of many people that the theory never really made sense to them.
Very interesting article Petra. As someone who has studied Aromatherapy and whether there is a pharmacological effect, one of the issues I have is that people extrapolate that applying an essencial oil directly onto a particular type of tissue will have the same effect as rubbing a few drops onto someone’s skin. There is a whole field of study with regards to trans dermal drug delivery and there are number of factors:
The drug has to be able to penetrate the skin barrier (i.e. lipophyllic), it then needs to be able to get into the blood supply to get the area of action (hydrophyllic) and once it reaches the receptor site, there has to be enough of it to have an effect – bio-availablity. None of these can be explained with FGT. Poul
As an Aromatherapy student who was only beginning to attempt to grasp FGT, this is mindblowing for me! I’m glad I am coming along at a time when a new framework for understanding is being developed, so I won’t have to “unlearn” FGT; yet the biological action of oils appears to be much more complex than I ever imagined. Chemistry was my weakest subject in college… I have a steep learning curve ahead!
Thank you, Petra ! Super-information ! It is great to see the limitations melting. I did not know you, but got to know Melanie Kovac when she came to our intl. Aromatherapy Center in Provence this spring. Would love to see you there at one of our courses next year !!
Yes, molecules are molecules – but what is “sitting” in between and behind is another question.
And yes ! We are entering a new era of field energy research. We know that there is a hidden network of electro-magnetic or quantum-field interactions everywhere, and – beyond that – the universal life force (PRANA) has its hands in it. Bio-chemical compounds are god to know but cannot stand for everything. But still useful and nice tools to play around with for a while….
But, as we also know, we approach new visions of reality step by step, and then we see that there are realities (plural) – then we see that we actually know very little, (or nothing) – and stop categorizing – rather just hinting, suggesting…..
….and this “cloud of unknowing” is not depressing, but rather encouraging.
Thanks again for everything ! Dr. Malte Hozzel
You are welcome Malte, and thank you for commenting! I agree very much that our knowledge is extremely limited, and that we often think we know a lot more than we actually do. It’s a thin line between what ‘works for me’, what fits one’s frame of understanding, and what may be common knowledge and experience. I’m aware of some of these theories, and they are indeed very fascinating. However, I think it’s crucial to be critical – especially in the fields such as aromatherapy with so many approaches – and to know how to distinguish between sound evidence, speculation, and obvious fails (such as FGT).